Atomic Theory, Periodic Classification and Properties of Elements notes
Introduction :-
- Man has been trying since ancient times to know about the substance.
- Ancient Indian and Greek philosophers were collecting information about the microscopic nature of substance since a long time (almost 500B.C.)
- The ancient Indian philosopher Maharishi Kanad told that when substance is continuously divided in small pieces then at last, smallest particles, atoms are obtained. It is not possible to further divide this smallest particle.
- An another Indian philosopher Pakudha katyayam told that different forms of substance are obtained from the combination of these particles.
- Almost at the same time, Greek philosopher Democritus and Leucippus called these smallest indivisible particles as atoms. It is taken from Greek word atomio which means indivisible.
- All these ideas about the atom were only philosophical, there was not any scientific or experimental basis. At the end of 18th century, many important works were done for it and atomic theories were given on the basis of laws and experimental facts.
Atomic theory of Dalton :-
- In 1808, a British school teacher, John Dalton gave a theory to explain atom.
- This atomic theory was given on the basis of chemical combination, mass conservation and law of constant proportion.
Its main postulates are :-
- Each substance is made up of small particles which are called atoms.
- Atoms are indivisible particles.
- All atoms of an element are same i.e. have same size, mass and chemical property.
- Atoms of different element have different mass, size and chemical property.
- Atoms of different element always combine in simple property of small whole number to form compound
- In chemical reaction, atoms only rearrange they can neither be created nor be destroyed by chemical reaction.
Drawback :-
- Atomic theory of Dalton could not explain facts but it laid strong foundation of advanced research about the atoms on the basis of scientific and experimental facts.
- Till the end of 19th century it was known that some more smaller particles are also present in atoms. Due to presence of these subatomic particles, atomic structure was further modified.
Atomic model of Thomson : -
- According to him, atom is a positively charged sphere of size 10౼¹⁰ m, in which negatively charged electrons are distributed in an equal amount.
- It is also called Plum Pudding Model.
- It is a type of Christmas cake in which positive charge is considered as pudding and electrons are like plum.
- In Indian perspective they can be considered as Bundi ka Laddu or water melon, Red part of water melon is like positive charge and seeds in between are like electrons.
- In this model, Thomson cleared that in an atom, amount of positive and negative charge is same and atoms are electrically neutral.

Drawback :-
- This model could not explain Rutherford gold foil experiment so this theory was declared invalid.
- It remained only of historical importance.
Rutherford's gold foil experiment : -
- This Models had been developed to understand the internal structure of these electrons and protons in an atom.
- First model related to atomic structure was given by Sir J.J. Thomson in year 1898.
Its main postulates are :-
- Ernest Rutherford and his students in 1911 did the experiment of bombarding particles on very thin gold foil.
- In this, highly energetic particles (nucleus of He ) were bombarded on thin gold foil (thickness 10౼¹⁰ m or 100 nm).
- A zinc sulphide coated circular plate was kept around foil so that after bombardment, particle strike on screen and produce flash.
- In this way, direction of particle was determined.
The observation of this experiment were :-
- Most of particles moved straight through the gold foil without deflection.
- A very few particles were deflected through small angle.
- Out of 20,000 particles, one particles was deflected by 180⁰ angle
The observations received from this experiment were unexpected. Rutherford himself stated that "It was almost as incredible as if you fired a 14 inch shell at a piece of tissue paper and it came back and hit you."

- Most of the part of atom is empty and changeless so most ∝ - particles pass straight without deflection
- Some ∝ - particles get deflected so it is confirmed that strong repulsive force act on them, so entire positive charge should be concentrated at a place in the atom.
- In an atom volume of positive charge is very less as compared to total volume. This positively charged volume was called nucleus the diameter of atom is almost 10౼¹⁰ m and diameter of nucleus is almost 10౼¹⁵ m.
On the basis of these conclusions, Rutherford represented the following model of an atom
- The entire positive charge and mass of the atom are concentrated at its centre called nucleus.
- Most of the part of the atom is empty in which electron move with high speed in circular path these circular paths are called orbits.
- 3.Atoms are electrically neutral. So it is confirmed that many electrons are there in an atom, as the number of protons are present in nucleus.
- Atomic model of Rutherford can be considered as solar system. In this model, electrons move around the nucleus in different orbits in the same way as different planets move in different orbits around the sun.
Limitations of Rutherford model : -
- It could not explain stability of an atom.
- It could not make clear the electronic structure of an atom.
Maxwell's theory :-
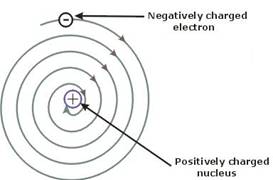
According to Maxwell's theory, electron moving in a circular path will emit radiation by which its energy will continuously decrease. In this way it will move in spiral motion and at the end it will fall in nucleus. But actually, it does not happen.
Drawback of Maxwell's theory : -
- It does not explain spectrum of atom.
- It does not explain number of electrons and their arrangement in an atom
Hypothesis of Neil's Bohr: -
- In 1913, Neil's Bohr proposed a model to explain structure and spectrum of hydrogen atom logically.
- Neil Bohr using quantum theory of physics, tried to remove the drawbacks of Rutherford model.
Bohr's atomic model is based on the following hypothesis :-

- In the centre of atom, nucleus is present which contain positively charged particles protons
- The electrons move around nucleus in paths of fixed radius and energy. These paths of fixed energy are called orbits or energy levels.
- These orbits are arranged concentrically around the nucleus. These are represented by n. Their value is always an integer like 1, 2. 3. 4 ...... and they are shown by K.L.M.N ....... respectively.
- With increase in value of n, orbits get away from nucleus and their energy increases, The orbit n = 1 or K has least energy. In these orbits, angular momentum of electron is mvr = h/2П or its multiple. Here h = Planck's constant, m = mass of electron, v = velocity of electron, r = radius of orbit
- According to Bohr, there is no change in energy of electron on revolving in a fixed orbit.
- When an electron absorbs energy from outside the atom, then gets excited and move to higher energy level.
- If electron emits energy then it transit from higher energy level to lower energy level.
- In an atom, due to absorption and emission of energy by the electron, the linear spectrum is formed.

- Bohr's atomic model was more developed as, linear spectrum of atom and its stability could be explained.
Limitations of Bohr model :-
- Atoms with large number of electrons could not be explained by this model.
- Through high resolving power apparatus was known that linear spectrum of an atom divided into more than one line. The reason could not be explained by Bohr model.
- It could not explain the process of formation of molecule by atom through chemical bond.
Necessity of classification :-
- Till 1800. Only 31 elements were known.
- Till 1865,63 elements had been discovered
- Today 118 elements (according to IUPAC) are known.
- although some of these elements are man - made.
- It is very difficult to remember all the elements, their physical and chemical properties and properties of the compounds formed by them individually. So the necessity of classification of elements was felt.
- Scientists tried to arrange these facts in a sequence based on some properties, so that they can be studied in a simple and rational number.
- By this type of classification, the study of new elements to be discovered in future will also be done in an organised way.
Classification :-
The classification of elements is the result of many years of experiments and hypothesis of scientists.
Dobereiner's Triads :-
- Firstly the name of Dobereiner comes in this series.
- In 1829. he made groups of 3-3 elements whose physical and chemical properties were the same, these were called Dobereiner's Triads.
- This group atomic weight of middle element was equal to the average of atomic weights of remaining two elements and properties were also in between their properties.

Drawback of Dobereiner's Triads :-
- Dobereiner found only three such triads. After that this law was not useful.
Newland's Law of octaves :-
- Then British chemist John Alexander Newland in 1865, gave Law of octaves.
- He arranged the elements in increasing order of their atomic weights and found that properties of 8 "element were similar to first one. like the nodes of music after seven notes , eighth note is the same as first note.
Drawback of Newland's Law of octaves :-
- This law also not proved useful for element after calcium
Mendeleef's periodic table :-

- The credit for making periodic table at first goes to Mendeleef.
- He arranged elements in increasing order of their atomic weight and saw that after a fixed interval same properties of elements repeats.
- Considering it as basic, he gave periodic law, "Properties of elements are periodic function of atomic weight".
- Mandeleef arrange periodic table in horizontal raw and vertical column. He called horizontal raw as a period and vertical column as a group.
- His table has 8 group which is divided into two sub-group A & B.
- Six period were made which again divided in series.
- Mendeleef arranged the elements in the table in the increasing order of atomic weight.
- He confirmed that elements with same type of physical and chemical properties should come in the same group, so that periodicity of elements is maintained.
- For it, somewhere he had to break the order of atomic weight. Like atomic weight of iodine (I) is 126.9 and tellurium (Te) is 127.6 but I is kept after Te because its properties resembles with properties of elements of VII group.
- In similar way, he left blank spaces for some elements in periodic table and did future predictions for them.
- He left gaps for Eka-boron, Eka-aluminum and Eka-silicon and predicted their properties which were proved correct later. Later they were called Scandium, Gallium and Germanium respectively.
- The formation of this table proved very useful and important in classification and study of elements.
Drawback of Mendeleef's periodic table :-
- At some places, increasing order of atomic weight was not followed.
- Some elements with similar properties were kept in different groups and some elements with dissimilar properties in same group.
- Hydrogen was not given fixed position.
- Isotopes were not given any position.
Modern periodic table :-
 |
| Modern period table |
- In the beginning of 20'h century, after the information about electron, proton and neutron had arrived, Henry Moseley (in 1913), rearranged periodic table.
- He found that atomic number represent the elements in periodic table better than atomic weight.
- Thus, he gave modified periodic law according to which, "Physical and chemical properties of elements are periodic function of their atomic number" It is called Modem Periodic law.
- In modern periodic table, elements are kept on the basis of increasing atomic number.
- In neutral atom, atomic number i.e. number of protons present in nucleus is same as total number of electrons present. So this periodic table itself represent the electronic configuration of elements.
- This form of periodic table is more simple and better detailed than Mendeleef periodic table. So it is also called extended or long form of periodic table.
- In this periodic table, horizontal rows are called periods and vertical columns are called groups.
- Number of groups are 18 and number of periods are from 1 to 7.
- Periods represent shell that means principle energy level n.
- First period have two elements. It is called very short period
- Second and third period have 8-8 elements, these are called short period.
- In fourth and fifth period, d-orbitals are also included. Both these periods have 18-18 elements. These are called long period.
- In sixth and seventh period, f-orbitals are also placed, so they have 32-32 elements, these are called very long period.
- In sixth and seventh period, f - orbitals are also placed, so they have 32 32 elements, these are called very long period.
- Although each element of f - block is written in two horizontal rows by 14-14 elements separately. In these, elements of first row are called lanthanides and elements of second row are called actinides.
- From this periodic table, it is clear that in same group or same vertical column, outermost electronic configuration of elements is same.
- In all these elements of a group, number of valence electrons which means number of electrons in outermost shell are same. In that group, on going down, only number of shells increases.
- On the basis of last electron filled in outermost shell, these elements can be divided in four blocks. Group 1 and 2 are called s-block elements, group 13 to 18 are p- Block elements, group 3 to 12 are d-block elements and at bottom both horizontal rows are called f-block elements.
- In horizontal rows, elements of first row (4f series) comes after lanthanum, so these are called lanthanides.
- Elements of second row (5f series) comes after actinium, so these are called actinides.
- Elements of s-block are called alkali or alkaline Earth metal, p-block are called principal elements, d-block are called transition elements and elements of f-block are called inner transition elements.
- In periodic table, elements after uranium are called ultra uranium (transuranic) elements.
- In this way, in periodic table, electropositive metallic elements are placed at left and electronegative non-metallic elements on the right.
- The zig-zag ladder like line below B, Si, As, Te and At make boundary between metal and non metals. These elements are called metalloids.
Periodicity in properties :-
- On the basis of periodic table, many physical and chemical properties of elements can be explained.
- If in periodic table, we go from left to right in a period or move down in a group then there is a definite order of increase or decrease of physical and chemical properties of elements.
- This regular change in properties of elements depends on their electronic configuration.
- In the periodic table, there is gradual change in electronic configuration of elements, with it gradual change in properties of elements can also be seen.
- This sequential change in properties is called periodicity in properties and properties are called periodic properties. Like atomic radius, melting point, boiling point, ionization enthalpy, valency etc.
- The force of attraction felt by electons of outermost shell of an atom by nucleus is called effective nuclear charge. Effective nuclear charge is always less than actual nuclear charge because mutual repulsion force between electrons present in outermost shell is balanced in small amount by nuclear attraction force. Effective nuclear charge is an important property by which periodicity of properties in periodic table is affected.
Atomic Radius :-
- The distance between nucleus and electron present in outermost shell of atom is called atomic radius. It is a very small unit.
- In a period, on moving from left to right, atomic number increases, thus number of proton in nucleus increases, so more nuclear attraction force act on electrons present in outermost shell, so the atomic radius decreases.
- On going from left to right in a period, effective nuclear charge increases and on moving down a group, it decreases.
- On moving down a group, atomic number increases, number of shells also increases and value of effective nuclear charge decreases. So, atomic radius increases.
Ionic radius :-
- When an atom accepts or donates electron then ion is formed Radius of ion is called ionic radius.
- When an atom donates electron, then positive ion is formed. In formation of positive ion (cation), with removal of electron, outermost shell of electron gets completely over and effective nuclear charge on remaining electrons increases. So size of cation is always less than neutral atom.

- When an atom accepts electron, then negative ion (anion) is formed. In formation of anion, number of electrons in outermost shell increases and value of effective nuclear charge decreases. So size of anion is always larger than size of neutral atom.
Ionisation enthalpy : -
- The energy given to isolate an electron from a neutral atom of an element in gaseous state is called ionisation enhalpy or ionisation potential.
- It is measured in calorie/mole or Kilo Joule/mole or electron volt/mole.
- As energy is given in this process so its value is always positive. Neutral atom + ionisation enthalpy ⟶ positive ion (g) + e-
- The energy given to separate first electron from neutral atom is called first ionisation enthalpy and energy given to isolate one more electron from cation is called second ionisation enthalpy. Similarly energy given to isolate third electron is called third ionisation enthalpy.
- Generally, for an element, first ionisation enthalpy (IE) < second IE < third IE.
- On going from left to right in a period, as atomic size decreases and value of effective nuclear charge increases, so it becomes difficult to isolate electron from the atom. The value of ionisation enthalpy increases.
- On moving down a group, number of shells increases, so atomic size increases and due to decrease in effective nuclear charge, attraction on outermost electrons becomes less. So it is easy to separate electron from neutral atom. On moving down a group, value of ionisation enthalpy of elements decreases.
Electron gain enthalpy : -
- It is also called electron affinity in gaseous state.
- When a neutral atom of any element accepts an electron, then negative ion (anion) is formed and the energy released is called electron gain enthalpy or electron affmity.
- Its value can be positive or negative, it depends on the nature of the element. Neutral atom(g) + e- ⟶ anion (g) + free energy (Electron gain enthalpy)
- On going from left to right in a period, atomic size decreases and effective nuclear charge increases, so value of electron gain enthalpy increases.
- On going down in a group, atomic size increases and effective nuclear charge decreases, so value of electron gain enthalpy decreases.
Electronegativity :-
- In covalent compounds, the property of attracting electron of chemical bond formed between two different atoms by an atom is called electronegativity.
- This is a relative tendency of elements.
- On moving from left to right in a period, due to decrease in atomic size, electronegativity of elements increases.
- On moving down a group, atomic size increases, so value of electronegativity decreases.
- The most electronegative element is fluorine (F).
Valency :-
- The number of electrons present in outermost shell of an element determines valency of that element. This property can be explained by electronic configuration of elements.
- Generally, the number of hydrogen atoms combining with an atom of an element or half of the number of oxygen atoms combining with an atom is called valency.
- Element of same group show same valency because electronic configuration of their outermost shell are same.
- In members of s-block i.e. group 1 and 2 there are 1 and 2 electrons respectively in the outermost shell and their valency is also 1 and 2 respectively.
- The valency of p-block elements or elements of group 13 to 17 is obtained by number of electrons present in its outermost shell or by subtracting the number of electrons present in outermost shell from 8.
- Generally, valency of 18th group is zero.
- On moving from left to right in a period, valency increases from 1 to 4 and then decreases. If element combines with oxygen then valency increases from 1 to 7.
- A part from this, d-block elements lanthanide and actinide elements show more than one valency, it is called Variable Valency. It is characteristic property of elements of this group.
- Now, oxidation state is used instead of valency. According to electronegativity, number of electrons or charge accepted by atom of an element from atom of another element, is called its oxidation state.
Atomic size :-
- Atomic size is a measurement of distance of nucleus of an independent atom from its outermost shell.
- It is very difficult to measure size of one atom of any element because these are found as molecule or group of atoms.
- It is very rare for an isolated atom to exist. Thus, atomic size is determined on the basis of radius of that atom. Radius of an atom can be in any form of the following. (i) Covalent radius (ii) Vanderwaals radius (iii) Metallic radius
- Covalent radius :-When two same atoms of an element are covalent bonded, then half of the distance between nuclei of both atoms is called covalent radius.
- Vanderwaals radius :-In solid state, half of the distance between atoms of two close non bonded molecules of same substance is called vanderwaal radius. Thus, value of vanderwaal radius is always greater than covalent radius.
- Metallic radius : - In crystal lattice of metal, half the distance between nucleus of two nearby atoms is called metallic radius.

- In periodic table, atomic size increases. On moving down a group as number of shells increase.
- On moving from left to tight in a period, number of electron in that shell increases and effective nuclear charge also increases, so atomic size decreases.
Metallic and non-metallic properties :-
- Metallic and non metallic properties of elements depend on this periodic property of atomic size.
- The tendency of an atom of any element to donate electron and form positive ion is called metallic property.
- Like alkali metals of group 1 are the most electropositive elements, because they easily donate electron and form positive ion. They possess highest metallic properties.
- The tendency of an atom of any element to accept electron and form negative ion is called non metallic property. Like the halogen group (group 17) elements easily accept an electron to form anion so they have strong non metallic properties.
- On moving down a group, size of atoms of element increases and value of effective nuclear charge decreases. Value of ionisation enthalpy sequentially decreases and formation of cation occurs easily.
- On moving from left to right in a period, atomic size of elements decreases and value of effective nuclear charge increases. Value of ionisation enthalpy sequentially increases and formation of cation do not occur easily. That means metallic properties of elements decreases. Metals have electropositive characters.
- On moving from left to right in a period, atomic size decreases and due to increase in effective nuclear charge, electron gain enthalpy increases. So tendency to form anion increase and non-metallic properties of elements increases.
- On going down the group, electron gain enthalpy decreases so the non-metallic properties decreases.
- Elements on left part of periodic table are rich in metallic properties, As we move to right, metallic properties decreases and non metallic properties increases Non-metals are electronegative i.e. they have electronegative characters.
- In this way, a zigzag line is formed in periodic table which separates metals and non metals. The elements near this line possess both type of properties and are called metalloids. These are Boron, Silicon, Germanium, Arsenic, Antimony, Tellurium and Polonium.
- Generally, oxides of metal oxides are basic and non-metals are acidic.

0 Comments